Evaluation of second IICT call: intensive preparations required
Funding for "Investigator initiated clinical trials" continues to be in high demand. But preparing applications requires intense preparations.
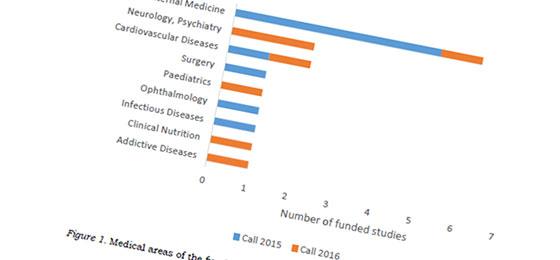
With the special programme Investigator Initiated Clinical Trials (IICT), the SNSF is promoting clinical studies in under-researched areas that do not attract industry interest. Projects of the first call in 2015 will be completed in 2019. The SNSF has now published the evaluation results of the 2016 call, listing the successful projects as well as the main reasons why the other proposals were rejected.
In response to the second call for proposals, 35 applications were submitted, of which 7 were selected for funding. This corresponds to a success rate of 20% (up from previously 12%). The 16 IICT projects of both calls are distributed across all Swiss university hospitals and involve in total 7300 patients from Switzerland and another 8 European countries. The overall budget of the ICCT programme amounts to CHF 22 million.
Experience so far shows that the preparation time needed for clinical studies up to the recruitment of patients is often underestimated. Sound planning requires great commitment on behalf of the study leader and good organisational structures. It therefore makes sense to secure the help of a study manager and to tap into the resources of local Clinical Trial Units (CTU). The evaluation results also show that, in addition to factors such as the high clinical relevance of the research questions, a convincing impact hypothesis and a good study design, a reliable calculation of patient numbers and evidence regarding feasibility are crucial.