The misunderstood massacre in the stomach
Doctors have demonised a germ in our stomachs because it’s carcinogenic. But it protected our forefathers from asthma and allergies. Could we still use its protection today? By Ori Schipper
Man’s most faithful servant is the dog, so they say. But our relationship to an inconspicuous microbe called Helicobacter pylori (H. pylori) goes back much further. Our forefathers carried this germ around with them 60,000 years ago when they left East Africa to explore and populate the rest of the world. This is proven by molecular-biological analyses of the genetic make-up of different strains of the microbe.
Biologists describe the microbe as a pathobiont, in other words an organism that can act as either a helpful guest in our stomachs or as a harmful pathogen. It is natural that the history of such a long relationship with so ambivalent a partner should be marked by trials and tribulations. And yet it is astonishing how vehemently medical science has changed its mind about this faithful microbe over the past thirty years.
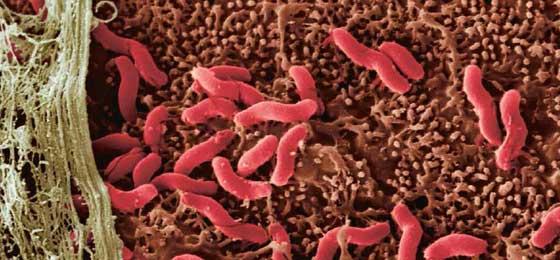
In the late 1970s, the Australian pathologist Robin Warren was regarded as an oddball. In stomach biopsies of gastritis patients that his colleagues at the clinic sent him, he found numerous, curved germs. At that time, the stomach was thought to be an organ that harboured no living bacteria. Science assumed that even the most resistant germs would be killed off by gastric acid. So Warren’s colleagues only grudgingly took note of his findings, assigning them no significance whatsoever.
The germ could be seen clearly on the photos that Warren made of his coloured specimens of stomach lining. But the gastroenterologists remained convinced that stomach upsets were connected to one’s lifestyle, and a result of too much stress or too much alcohol. They preferred to talk of ‘idiopathic gastritis’, i.e., of a stomach inflammation of unclear origin, instead of believing that Warren’s germ could have any causal relationship to inflammation in the stomachs of their patients.
But Warren’s ideas gained currency when the young, recently qualified gastroenterologist Barry Marshall joined him, having been assigned to “that pathologist who wanted to believe that gastritis was an infection”, as Warren remarked in 2005 when he and Marshall were awarded the Nobel Prize for Medicine. In 1982 Marshall took a small piece of normal, non-inflamed stomach lining from 100 patients who had visited him for a gastroscopy. Warren looked at the tissue samples under the microscope – and found the curved germ in more than half of them. In some patients, the presence of H. pylori went hand in hand with frequent belching, bad breath and ulcers in their duodenum, the first section of the digestive tract that follows the pylorus, the muscular opening from the stomach to the intestine.
A drastic self-experiment
Marshall racked his brains as to how to kill off these germs. Would the ulcers in the stomach and intestine just return afterwards? He achieved astonishing results with antibiotics. But the medical profession still wouldn’t change its mind. His sceptical colleagues lacked the final proof that the stomach germ actually caused stomach ulcers and was not just an attendant symptom of them. The germ isolated from a sick person had to be able to trigger the same illness in a healthy person who acquired it.
Because he made no progress with animal experiments, Marshall resorted to a last, drastic measure. He used himself as a guinea pig, swallowing a culture of H. pylori that he had grown from the stomach contents of one of his patients. After three days his breath began to smell badly. A week later he began vomiting repeatedly, and a biopsy of his stomach proved that the experiment had worked: Marshall had acquired gastritis.
In the following years it was proven that H. pylori covers itself in a protective coating that is able to neutralise stomach acid on the spot. And when other studies confirmed the results of Warren and Marshall, everyone gradually became convinced that this ‘impossible’ germ did indeed exist in the stomach. More than that: it’s dangerous. It is connected not just to ulcers of the stomach and intestines but also to stomach cancer, which is why the World Health Organization declared it to be a carcinogen in 1994.
This faithful microbe was now combatted intensively as a new enemy – and with success. The frequent prescription of antibiotics, and other factors such as clean drinking water and increased hygiene, led to fewer and fewer people having the germ in their stomachs. Whereas 50 years ago a large majority of humans had the germ (as do many still in large parts of Africa and South America), it is found today only in 10% of children in the USA and Europe.
As the germ has gradually disappeared, the rate of stomach cancer has also decreased. That is grounds for satisfaction – but this joy is increasingly tinged with regret. For several years there have been hints that the lack of the microbe can also have negative consequences. “H. pylori has two faces”, says Anne Müller of the Institute of Molecular Cancer Research at the University of Zurich. She and her team infected mice with the bacterium at two different times: just after birth for one group, and six weeks later for the other. The immune system of the mice infected early was not yet fully developed and was thus ‘tolerogenic’. In other words, the immune system believed that the microbe belonged to it and so wasn’t a target for attack. As a result, these mice had a hundredfold more germs in their stomach than those that were infected later. But, astonishingly, they had no stomach problems.
Power of conviction
When mice are infected at six weeks, the picture is very different. Their adult, mature immune system reacts immunogenically: it regards H. pylori as an intruder that has to be attacked. But the immune system cannot win the battle, so it fails to wipe out the germ and a certain number of H. pylori cells remain in the stomach of the mice. These then trigger chronic inflammation.
“It’s not the microbe itself, but the chronic defensive reaction of our body that carries out this massacre in the stomach” says Müller. Her group has discovered that the microbe in the stomach is able to influence our immune system and reacts in an ‘immunomodulatory’ fashion. The microbe convinces our immune system to offer a ‘youthful’, tolerogenic answer. This is why the adult system cannot react so as to kill off the germ completely. It’s not that the attack of the immune system stops, it is just redirected against the cells of the body’s own gastric mucous membrane. This ultimately degenerates into an ulcer or even into cancer.
H. pylori has adapted to life with man over the course of thousands of years and has learnt to train our immune system not to sound the alarm against all germs in the body. For this reason, the significance of this microbe extends beyond the realm of stomach problems. Over the last 30 years, coincident with the reduction in H. pylori, scientists have noticed a sharp increase in allergic illnesses. Other tests carried out by Müller’s group have proven that this development did not just happen by chance at the same time, but that there is a causal relationship involved. The stomach germ when given early to mice also protects them from asthma, hay fever, neurodermatitis and coeliac disease throughout their lives, for example. “This complete protection is the most drastic phenotype that I ever had the pleasure of investigating”, says Müller.
Müller can achieve a lot with the “disappearing microbiota hypothesis”. It posits that the loss of microbes inherited from our distant forefathers is connected to numerous diseases in modern society, such as obesity or asthma, that have beset the northern hemisphere most of all in the last thirty years. If we were to resort less often to using antibiotics – especially in children – and thereby better preserve the “microbiome of our forefathers”, then we could utilise various germs that make our immune system more tolerant, according to Müller. “We should not rid ourselves of useful microbes without good reason”.
But H. pylori is a complex case. The gastroenterologists actually have good grounds for their efforts to eradicate it. “The germ has a bad reputation, and rightly so. Cancer is worse than asthma, after all. There is no question of prescribing living microbes for therapeutic purposes”, says Müller. She and her team are pursuing a more subtle approach.
Children with asthma
In H. pylori, they have identified two so-called persistence factors, i.e., the molecules that the germ excretes and that make the immune system tolerogenic or benevolent. Müller and her group have tested whether these two factors in isolation would suffice for protection against asthma. “It functions astonishingly well in mice”, says Müller. Now, in collaboration with the pharmaceutical industry, they are developing a new inoculation strategy with which she hopes to avoid the disadvantages of the germ, without losing its advantages. She can imagine using it to treat children with a high risk of asthma. The persistence factors could help to avoid the threat of stomach cancer, yet still utilise the valuable immunomodulatory qualities that H. pylori has acquired in the course of its long, common history with mankind.
Ori Schipper is a science editor at the SNSF.
(From "Horizons" No. 102, September 2014)